Products
A company specializing in the development, production and sales of in vitro diagnostic products.
Product classification
Contact Us
Phone/WeChat:
E-mail:
info@immuno-test.com
Address:
Building 1, 578 No.20 Street, Qiantang District, Hangzhou City, Zhejiang Province
Zika Virus IgG/IgM Rapid Test
Phone:0571-85368996 | Email:info@immuno-test.com
Abbr.:
Specification:
Specimen:
Assay Time:
Model No.:
HZIK009T
Model No.: HZIK009T
Assay Time: 10-15 minutes
Specimen: Whole Blood,serum or plasma
Zika Virus IgG/IgM Rapid Test
Graphic Details
INTENDED USE
The Zika virus IgG/IgM Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to Zika virus in human whole blood, serum, or plasma as an aid in the diagnosis of primary and secondary Zika infections.
| [Product Name] | Zika virus IgG/IgM Rapid Test |
| [Assay Time] | 10-15 minutes |
| [Specimen] | whole blood, serum or plasma specimen. |
| [Principle] | Rapid chromatographic immunoassay |
| [Storage Temperature] | 2-30℃,DO NOT FREEZE |
| [Usage] | For the detection of IgG and IgM Zika antibodies in human whole blood, serum, or plasma. |
| [Packing] | 20 tests/box |
| [Materials Provided] | Individually packed test devices |
| Package insert |
|
| Disposable pipettes |
|
|
Buffer |
PRINCIPLE
The Zika Virus IgG/IgM Rapid Test is a qualitative membrane - based immunoassay for the detection of Zika antibodies in whole blood, serum, or plasma.
TEST PROCEDURE
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15 - 30°C) prior to testing.
1. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
Hold the dropper vertically, draw the specimen up to the Fill Line (approximately 10 μL), and transfer the specimen to the specimen well (S) of the test device, then add 2 drops of buffer (approximately 80 μL) and start the timer.
For Whole Blood (Venipuncture/Fingertick) Specimens:
To use a dropper: Hold the dropper vertically, draw the specimen 0.5 - 1 cm above the Fill Line, and transfer 2 drops of whole blood (approximately 20 μL) to the specimen well (S) of the test device, then add 2 drops of buffer (approximately 80 μL) and start the timer. See illustration below.
To use a micropipette: Pipette and dispense 20 μL of whole blood to the specimen well (S) of the test device, then add 2 drops of buffer (approximately 80 μL) and start the timer.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
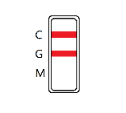
IgG POSITIVE: The colored line in the control line region (C) appears, and a colored line appears in test line region G. The result is positive for Zika virus specific-IgG and is probably indicative of secondary Zika infection.

IgM POSITIVE: The colored line in the control line region (C) appears, and a colored line appears in test line region M. The result is positive for Zika virus specific-IgM antibodies and is indicative of primary Zika infection.
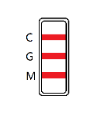
IgG AND IgM POSITIVE: The colored line in the control line region (C) appears, and two colored lines should appear in test line regions G and M. The color intensities of the lines do not have to match. The result is positive for IgG & IgM antibodies and is indicative of secondary Zika infection.
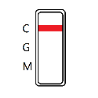
NEGATIVE: The colored line in the control line region (C) appears. No line appears in test line regions G or M.
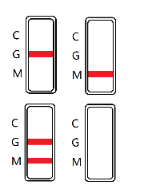
INVALID: No Control line(C) appears.
Previous Page
Next Page
Previous Page
Next Page
Related Products
Online message