Products
A company specializing in the development, production and sales of in vitro diagnostic products.
Product classification
Contact Us
Phone/WeChat:
E-mail:
info@immuno-test.com
Address:
Building 1, 578 No.20 Street, Qiantang District, Hangzhou City, Zhejiang Province
SARS-CoV-2 Antigen Rapid Test (Nasal Swab)
Phone:0571-85368996 | Email:info@immuno-test.com
Abbr.:
Specification:
Specimen:
Assay Time:
Model No.:
HCOV017G
Model No.:HCOV017G
Assay Time:10-15 minutes
Specimen:Nasopharyngeal swab, or oropharyngeal swab specimen
SARS-CoV-2 Antigen Rapid Test (Nasal Swab)
Graphic Details
INTENDED USE
The SARS-CoV-2 Antigen Rapid Test (Nasal Swab) is a rapid chromatographic immunoassay for the qualitative detection of novel coronavirus SARS-CoV-2 in human nasal swab specimen.
| [Product Name] | SARS-CoV-2 Antigen Rapid Test (Nasal Swab) |
| [Product No.] | HCOV017G |
| [Assay Time] | 10-15 minutes |
| [Specimen] |
Nasopharyngeal or oropharyngeal secretion |
| [Principle] | Rapid chromatographic immunoassay |
| [Storage Temperature] | 2-30℃,DO NOT FREEZE |
| [Usage] | The SARS-CoV-2 Antigen Rapid Test is for detection of SARS-CoV-2 antigens. |
| [Packing] | 20 tests/box |
| [Materials Provided] | Foil pouches, each contains one test cassette, and one desiccant bag |
| Assay buffer tubes (0.5ml each) and tips |
|
| Disposable Sampling Swabs |
|
|
Positive control swab and negative control swab |
|
| Instruction for use |
PRINCIPLE
The SARS-CoV-2 Antigen Rapid Test is for detection of SARS-CoV-2 antigens. Anti-SARS-CoV-2 monoclonal antibodies are coated in the test line and conjugated with the colloidal gold. During testing, the specimen reacts with the anti-SARS-CoV-2 antibodies conjugate in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with another Anti-SARS-CoV-2 monoclonal antibodies in the test region. The complex is captured and forming a colored line in the Test region. The SARS-CoV-2 Antigen Rapid Test contains anti-SARS-CoV-2 monoclonal antibodies conjugated particles and another anti-SARS-CoV-2 monoclonal antibodies are coated in the test line regions.
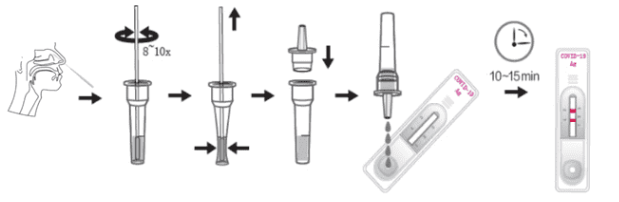
SPECIMEN COLLECTION
Nasal Swab: Insert the sterile swab into one of the patient's nostrils around 2.5cm. Gently rub against the anterior nasal wall for 5 times. Take out the swab, and repeat the collection into the other nostril.
Take out of an assay buffer tube, and tear off the seal of the tube. Insert the swab into the tube and squeeze the flexible tube for 8~10 times to extrude the specimen from the head of the swab. Make the specimen resolved in the assay buffer sufficiently. Add the tip onto the assay buffer tube.
TEST PROCEDURE
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15 - 30°C) prior to testing. Run a quality control testing before using the kit (if necessary) according to the quality control protocols.
1. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and horizontal surface. Reverse the specimen collection tube, extrude 3 drops of the prepared specimen into the specimen well (S) of the test cassette and start the timer.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 15 minutes.
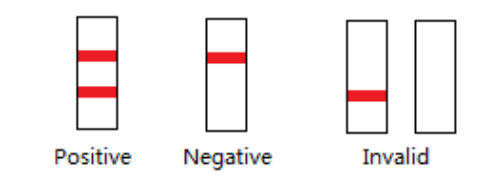
INTERPRETATION OF RESULTS
Previous Page
Related Products
Online message