Products
A company specializing in the development, production and sales of in vitro diagnostic products.
Product classification
Contact Us
Phone/WeChat:
E-mail:
info@immuno-test.com
Address:
Building 1, 578 No.20 Street, Qiantang District, Hangzhou City, Zhejiang Province
Respiratory Syncytial Virus Antigen Rapid Test
Phone:0571-85368996 | Email:info@immuno-test.com
Abbr.:
Specification:
Specimen:
Assay Time:
Model No.:
HRSV022G
Model No.:HRSV022G
Assay Time:15-20 minutes
Specimen:Nasopharyngeal swab, or oropharyngeal swab specimen
Respiratory Syncytial Virus Antigen Rapid Test
Graphic Details
INTENDED USE
The Respiratory Syncytial Virus Antigen Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Respiratory Syncytial Virus (RSV) antigen in human nasopharyngeal swab. The test results are intended to aid in the diagnosis of Respiratory Syncytial Virus infection in people.
| [Product Name] | Respiratory Syncytial Virus Antigen Rapid Test |
| [Product No.] | HRSV022G |
| [Assay Time] | 15-20 minutes |
| [Specimen] |
human nasopharyngeal swab, or oropharyngeal swab specimen |
| [Principle] | Rapid chromatographic immunoassay |
| [Storage Temperature] | 2-30℃, DO NOT FREEZE |
| [Usage] | Designed as a simple tool in detection of RSV for regular clinical diagnosis. |
| [Packing] | 20 tests/box |
| [Materials Provided] | Individually packed test devices |
| Workstation |
|
| Buffer tubes with dropper tips |
|
|
Package insert |
|
| Disposable Sampling Swabs |
PRINCIPLE
The Respiratory Syncytial Virus Antigen Rapid Test is consist with one test strips which could be observed in the window of the rapid test device. The strip is based on sandwich method immunochromatographic assay.
SPECIMEN PREPARATION
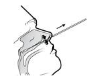
1. Insert the sterile swab into the deep nasal cavity until the nasopharynx. Gently rub and rotate the swab against wall of turbinate for several times.
2. Take out a assay buffer tube and unscrew the cap of the tube. Insert the swab into the tube and squeeze the flexible tube for 8~10 times to extrude the specimen from the swab tip. Make the specimen resolved in the assay buffer sufficiently and tighten the cap onto the assay buffer tube.
TEST PROCEDURE
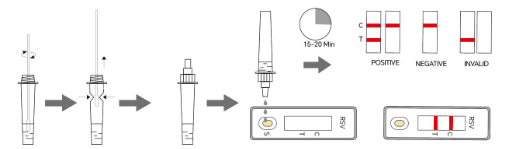
Allow the test device, specimen, buffer, and/or controls to reach room temperature (10 - 30°C) prior to testing.
1. Remove the test device from the foil pouch and use it within one hour.
2. Place the test device on a clean and horizontal surface. Reverse the assay buffer tube, extrude 3 drops (approx. 70μL) of the prepared specimen into the specimen well (S) of the test device and start the timer.
3. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
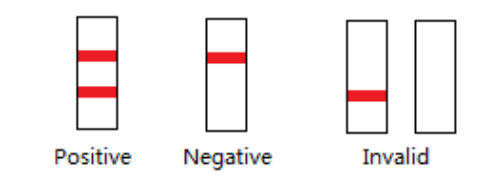
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears in the control region(C). No apparent colored band appears in the test region (T).
INVALID: Control band fails to appear.
Previous Page
Next Page
Previous Page
Next Page
Related Products
Online message